Posted on: Tuesday, 30 November 2010, 15:34 CST
SAN DIEGO, Nov. 30, 2010 /PRNewswire/ — Prometheus Laboratories Inc., a specialty pharmaceutical and diagnostic company, announced today that it has extended its distribution agreement with AstraZeneca LP for the exclusive marketing, sales and distribution of ENTOCORT® EC (budesonide) Capsules in the United States until December 31, 2011. ENTOCORT EC is the only FDA-approved drug for the induction and maintenance of clinical remission in mild to moderate Crohn’s disease involving the ileum and/or the ascending colon. ENTOCORT EC is indicated for the treatment of mild to moderate active Crohn’s disease involving the ileum and/or the ascending colon (up to 8 weeks with repeated 8-week courses as necessary for recurring episodes of active disease), and the maintenance of clinical remission of mild to moderate Crohn’s disease involving the ileum and/or the ascending colon for up to 3 months. Clinical remission is defined as a Crohn’s Disease Activity Index (CDAI) score of < 150.
“We are extremely pleased that we are able to continue to build upon our Gastroenterology franchise with our promotion and distribution of ENTOCORT EC through 2011,” said Joseph M. Limber, President and Chief Executive Officer of Prometheus. “Prometheus’ long-standing commitment to help patients suffering from Crohn’s disease manage their disease includes ENTOCORT EC, our IBD Serology 7 diagnostic test, as well as our recently launched proprietary Crohn’s Prognostic test, the first ever serogenetic prognostic test for Crohn’s disease.”
The original distribution agreement included a six-year exclusive term ending December 31, 2010. AstraZeneca will continue to supply the product to Prometheus and be responsible for all manufacturing, clinical and regulatory processes relating to the product. AstraZeneca launched ENTOCORT EC Capsules in November 2001.
ENTOCORT EC is the only FDA-approved drug for the induction and maintenance of clinical remission in mild to moderate Crohn’s disease involving the ileum and/or the ascending colon. ENTOCORT EC consists of an encapsulated formulation of budesonide granules, a glucocorticosteriod. the granules are coated to protect dissolution in gastric juice, but dissolve at pH >5.5, normally when the granules reach the duodenum. thereafter, a matrix controls the release of the drug into the intestinal lumen in a time-dependent manner. eighty to ninety percent of ENTOCORT EC does not enter the systemic circulation. ENTOCORT EC may reduce the incidence of some corticosteroid-associated side effects such as acne and moon face compared to prednisolone.(1)
Important Safety Information
Since ENTOCORT® EC (budesonide) Capsules is a glucocorticosteroid (GCS), general warnings about GCSs should be followed. GCSs can reduce the response of the hypothalamus-pituitaryadrenal axis to stress. Supplementation with a systemic GCS is recommended before surgery or other stress situations.
Adrenocortical function monitoring may be required in patients being transferred to ENTOCORT EC from a systemic GCS, and the dose of the systemic GCS should be reduced cautiously.
Patients on drugs that suppress the immune system are more susceptible to infections, which may be more severe, and should avoid exposure to infections such as chicken pox or measles.
Caution should be taken in patients with tuberculosis, hypertension, diabetes mellitus, osteoporosis, peptic ulcer, glaucoma or cataracts, or with a family history of diabetes or glaucoma, or with any other condition where GCSs may have unwanted effects.
Reduced liver function affects the elimination of GCSs, and increased systemic availability of oral budesonide has been observed in patients with liver cirrhosis. Patients with moderate to severe liver disease and patients who are concomitantly taking ketoconazole or any other CYP3A4 inhibitor should be closely monitored for increased signs and/or symptoms of hypercorticism. Reduction in the dose of ENTOCORT EC should be considered in these patients. Patients should be advised to avoid consuming grapefruits and grapefruit juice while being treated with ENTOCORT EC.
Safety and effectiveness in pediatric, geriatric, and pregnant patients have not been established. ENTOCORT EC should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy.
Budesonide is secreted in human milk. A decision should be made whether to discontinue nursing or to discontinue ENTOCORT EC, taking into account the clinical importance of ENTOCORT EC to the mother and the potential for serious adverse reactions in the nursing infant.
The adverse event profile of ENTOCORT EC in 6 mg once daily clinical trial treatment (52-week) was similar to that of 9 mg daily clinical trial treatment (8-week). the most frequently reported adverse events in clinical trials with ENTOCORT EC were headache, respiratory infection, nausea, and symptoms of hypercorticism.
Please see full Prescribing Information for ENTOCORT EC.
About Crohn’s Disease
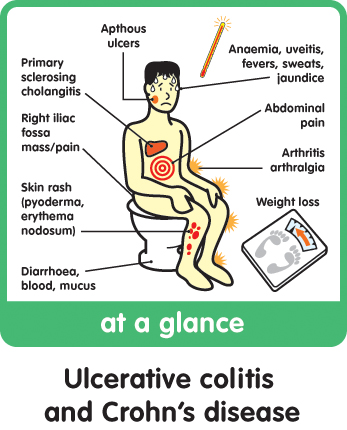
Crohn’s disease is an inflammatory bowel disease. it is a chronic disorder that causes inflammation of the digestive or gastrointestinal tract. the inflammation caused by Crohn’s disease is usually found in a part of the small intestine called the ileum and in the large intestine (colon). Symptoms of the disease include diarrhea, abdominal pain, fever, rectal bleeding, appetite loss, and weight loss. Symptoms may range from mild to severe. there is no known cure for Crohn’s disease. Crohn’s patients may require long-term medical care, including multiple hospitalizations, surgeries and therapeutics. the condition can be difficult to manage clinically.
About Prometheus
Prometheus Laboratories Inc. is committed to improving lives through the development and commercialization of novel pharmaceutical and diagnostic products that enable physicians to provide greater individualized patient care. Prometheus is a leader in applying the principles of personalized medicine to the diagnosis and treatment of gastrointestinal diseases and is applying these principles to oncology. Its strategy includes the marketing and delivery of pharmaceutical products complemented by proprietary diagnostic testing services. By integrating therapeutics and diagnostics, Prometheus believes it can provide physicians with more targeted solutions to optimize care for their patients. Prometheus’ corporate offices are located in San Diego, California.
(1) ENTOCORT® EC (budesonide) Capsules Prescribing Information
SOURCE Prometheus Laboratories Inc.
More News in this Category