 February 14, 2011 09:00 AM Eastern Time
February 14, 2011 09:00 AM Eastern Time
EWING, N.J.–(BUSINESS WIRE)–Antares Pharma, Inc. (NYSE Amex: AIS) today announced the receipt from the U.S. Food and Drug Administration (FDA) of a waiver, on February 8, 2011, for the $1,500,000 New Drug Application (NDA) filing fee for Anturol® Gel for overactive bladder (OAB).
“We are pleased to have received the waiver for the NDA filing fee for the Anturol NDA. this waiver represents a significant accomplishment for the Company and helps maintain our strong cash position”
The waiver, requested by Antares in accordance with section 736(d)(1)(D) of the Federal Food, Drug and Cosmetic Act, is granted to a small business for the first human drug application that it submits to the FDA for review. To be considered for the waiver, a company must demonstrate it meets the size restrictions established by the Small Business Administration (SBA), which the SBA confirmed for Antares on January 14, 2011. The FDA will typically provide notice of acceptance of the NDA within 60 days of receipt of the waiver.
“We are pleased to have received the waiver for the NDA filing fee for the Anturol NDA. this waiver represents a significant accomplishment for the Company and helps maintain our strong cash position,” said Paul K. Wotton Ph.D., President and Chief Executive Officer.
About Overactive Bladder
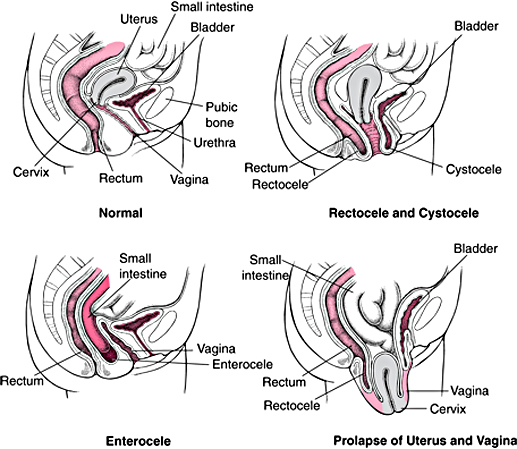
OAB, also called urge incontinence, is a condition marked by a sudden need to urinate that can happen at any time whether or not the bladder is full. OAB is typically caused when the smooth muscle of the bladder undergoes involuntary contractions and may result in uncontrolled leakage. OAB is defined as urgency, with or without urge incontinence and usually includes frequency and nocturia (waking up one or more times during the night to urinate). According to published reports it is estimated that more than 30 million Americans have OAB, and while it can happen at any age is more prevalent among older individuals. it is more common than both diabetes and asthma. According to IMS the annual OAB prescription market in the United States is valued at approximately $2.0 billion.
About Anturol®
Anturol is oxybutynin gel based on the ATD Gel technology platform which is a clear, odorless, hydroalcoholic gel that provides for delivery of oxybutynin in a non-patch transdermal form. The ATD technology is also used in Elestrin®, an FDA approved product for hormone replacement therapy in postmenopausal women. it has been well recognized that transdermal delivery of drugs including oxybutynin is a safe and effective way of delivering certain drugs that undergo first pass metabolism. by delivering oxybutynin transdermally, first-pass gastric and hepatic metabolism is avoided, which is believed to result in lower anticholinergic side effects such as dry mouth and constipation compared to orally administered treatments. These side effects account for a significant level of patient non-compliance among existing oral OAB treatments.
About Antares Pharma
Antares Pharma focuses on self-injection pharmaceutical products and topical gel-based medicines. The Company’s subcutaneous and intramuscular injection technology platforms include VIBEXTM disposable pressure-assisted auto injectors, ValeoTM/VisionTM reusable needle-free injectors, and disposable multi-use pen injectors. In the injector area, Antares Pharma has a multi-product deal with Teva Pharmaceutical Industries, Ltd that includes Tev-Tropin® human growth hormone and a partnership with Ferring Pharmaceuticals. In the gel-based area, the Company’s lead product candidate is Anturol®, an oxybutynin ATD™ gel that has completed Phase 3 studies for the treatment of OAB (overactive bladder). Antares also has a partnership with BioSante that includes LibiGel® (transdermal testosterone gel) in Phase 3 clinical development for the treatment of female sexual dysfunction (FSD), and Elestrin® (estradiol gel) indicated for the treatment of moderate-to-severe vasomotor symptoms associated with menopause, and currently marketed in the U.S. Antares Pharma has corporate headquarters in Ewing, New Jersey, with subsidiaries performing research, development and product commercialization activities in Minneapolis, Minnesota and Muttenz, Switzerland.