Patients with a rare deadly cancer have been offered a lifeline which can double their chances of survival. Skip related content
The drug sunitinib is the first licensed treatment for pancreatic neuroendocrine tumours (NET) in 25 years.
European regulators approved sunitinib for the disease after trial findings showed it increased survival time from 5.5 months to 11.4 months without worsening symptoms.
Full data on overall survival are not yet available. but the trend so far shows that patients on sunitinib have less than half the death rate of those given a placebo.
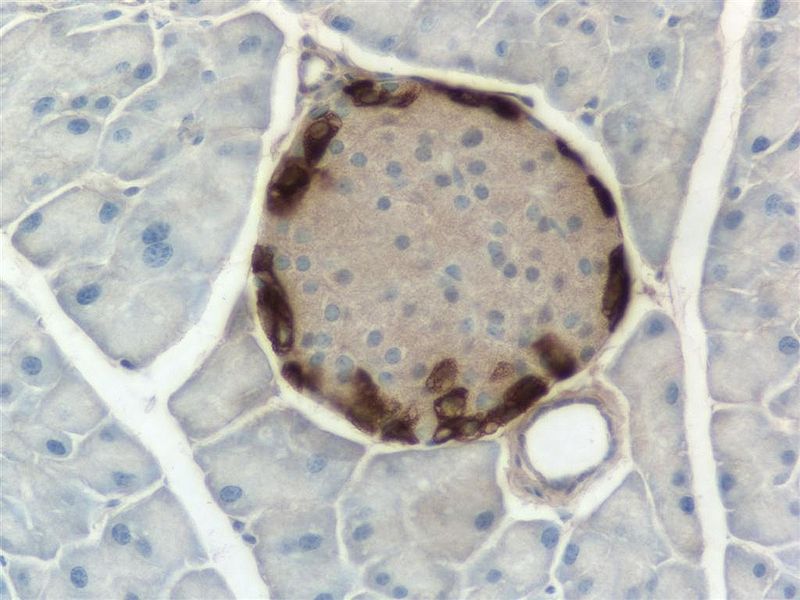
Pancreatic NET develops in the insulin-producing islet cells of the pancreas. the disease affects up to 240 people in the UK each year, typically between the ages of 40 and 60.
Dr Juan Valle, medical oncologist at the University of Manchester and Christie NHS Foundation Trust, said: "after years of working with few treatment options, sunitinib provides new hope for patients living with this difficult-to-treat disease."
Sunitinib, sold under the brand name Sutent, is already licensed in the UK for the treatment of advanced kidney cancer.